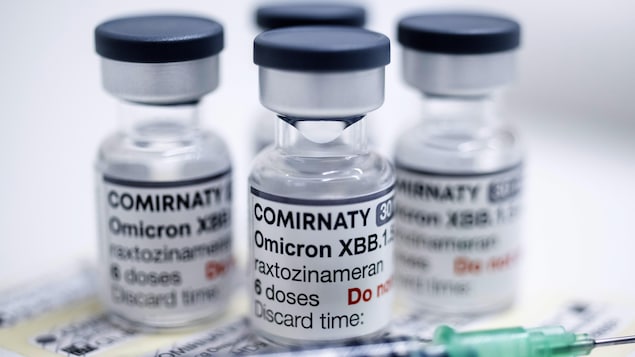
Authorization of the Pfizer-BioNTech Comirnaty vaccine, announced on September 28, targets the Omicron XBB.1.5 subvariant of the virus.
For children over five years old, the newer vaccine will be given as a single dose. For children aged six months to five years who have not yet received the primary series of shots, they must receive three doses of the vaccine.
Pfizer Canada says it expects new formulation of vaccine to be available in the next few weeks
ahead of the predicted peak of the respiratory virus season.
An updated vaccine from pharmaceutical company Moderna was approved earlier this month.
Dr. Bonnie Henry, B.C.’s chief provincial health officer, said Thursday that new and updated vaccines are showing good protection
Targets all Omicron subvariants currently in circulation. This is good news
he added.
Henry said the rapid antigen tests that are available also continue to work.

The new vaccine is not considered a booster vaccine against Covid-19, but rather a newer vaccine.
Photo: (Christopher Ladd/AP)
Canada will have sufficient supply of new mRNA vaccine formulation by fall 2023
Health Canada reported in a statement.
Health authorities are also reviewing the latest vaccine proposed by pharmaceutical company Novavax for people over 12 years old.
Federal officials not calling for new vaccine strengthen
. Instead, they consider them a newer option similar to the annual flu shot.
The latest report from the Public Health Agency of Canada for the week ending September 23 shows an increase in positive tests for the virus that causes Covid-19 since early July.
The agency also said activity of common cold viruses (enteroviruses and rhinoviruses) is increasing, as expected this time of year.
Source: CBC/A. Zafar
Adaptation: RCI / R. Valencia