by chala
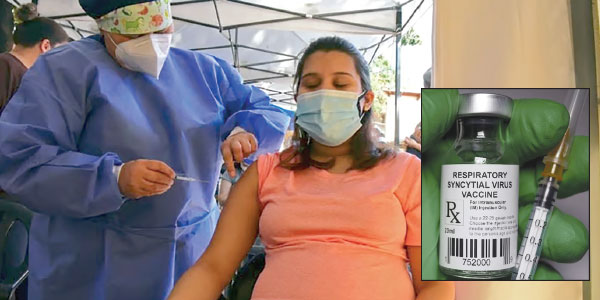
Vaccines to be injected during pregnancy
The U.S. Food and Drug Administration (FDA) announced on August 21 that it has approved Abrysvo, a vaccine given to pregnant women to help protect babies from illnesses related to respiratory syncytial virus (RSV).
The FDA press release quoted Dr. Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research, as saying, “This approval provides health care providers and pregnant women with an option to protect their babies from this potentially life-threatening disease.” .
Although RSV can infect people of any age, people between birth and 2 years of age are most likely to become infected with this contagious virus, according to information from the FDA. One way it can be life-threatening is by causing serious illnesses like bronchitis and pneumonia, according to the FDA.
To reduce the risk of infection, Abrysvo should be taken between the 32nd and 36th weeks of pregnancy, the FDA said.
The vaccine’s approval follows testing of its safety and effectiveness. The FDA said a study of about 3,500 pregnant women showed that the chance of severe lower respiratory tract illness in babies fell by 81.8% between birth and 60 days of age and by 69.4% between birth and 180 days of age.
As with other vaccines, there are potential side effects from taking Abrysvo. Symptoms cited by the FDA include headache and nausea.
Anyone interested in receiving the vaccine is advised to consult a health care professional.
FDA approves RSV vaccine
The vaccine will be given during pregnancy
The U.S. Food and Drug Administration (FDA) announced on August 21 that it has approved Abrysvo, a vaccine given to pregnant women to help protect infants from illnesses related to respiratory syncytial virus (VSR).
“This approval provides health care providers and pregnant women with an option to protect their babies from this potentially fatal disease,” Dr. Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research, said in an FDA news release.
Although RSV can infect people of any age, people between birth and 2 years of age are most likely to become infected with this contagious virus, according to the FDA. One way it can be life-threatening is by causing serious illnesses like bronchitis and pneumonia, according to the FDA.
To reduce the risk of infection, Abrysvo should be taken between the 32nd and 36th weeks of pregnancy, the FDA said.
The vaccine was approved after testing for safety and effectiveness. The FDA said a study of about 3,500 pregnant women showed that the chance of babies suffering from severe lower respiratory tract illness fell by 81.8% within 60 days of birth and by 69.4% within 60 days of birth. There was a 69.4% decrease within 180 days after birth.
As with other vaccines, there are possible side effects from taking Abrysvo. Symptoms cited by the FDA include headache and nausea.
Anyone interested in receiving the vaccine is advised to consult a healthcare professional.
