© marina_ua stock.adobe.come
Nonalcoholic steatohepatitis (NASH) is a serious liver disease that affects millions of people worldwide, including approximately 5.3% of adults worldwide and 14% of middle-aged adults in the United States. Although this form of NASH is common, there are currently no FDA-approved treatment options. Due to the lack of approved drugs, many patients look for alternative solutions and hope for breakthroughs in medical research.
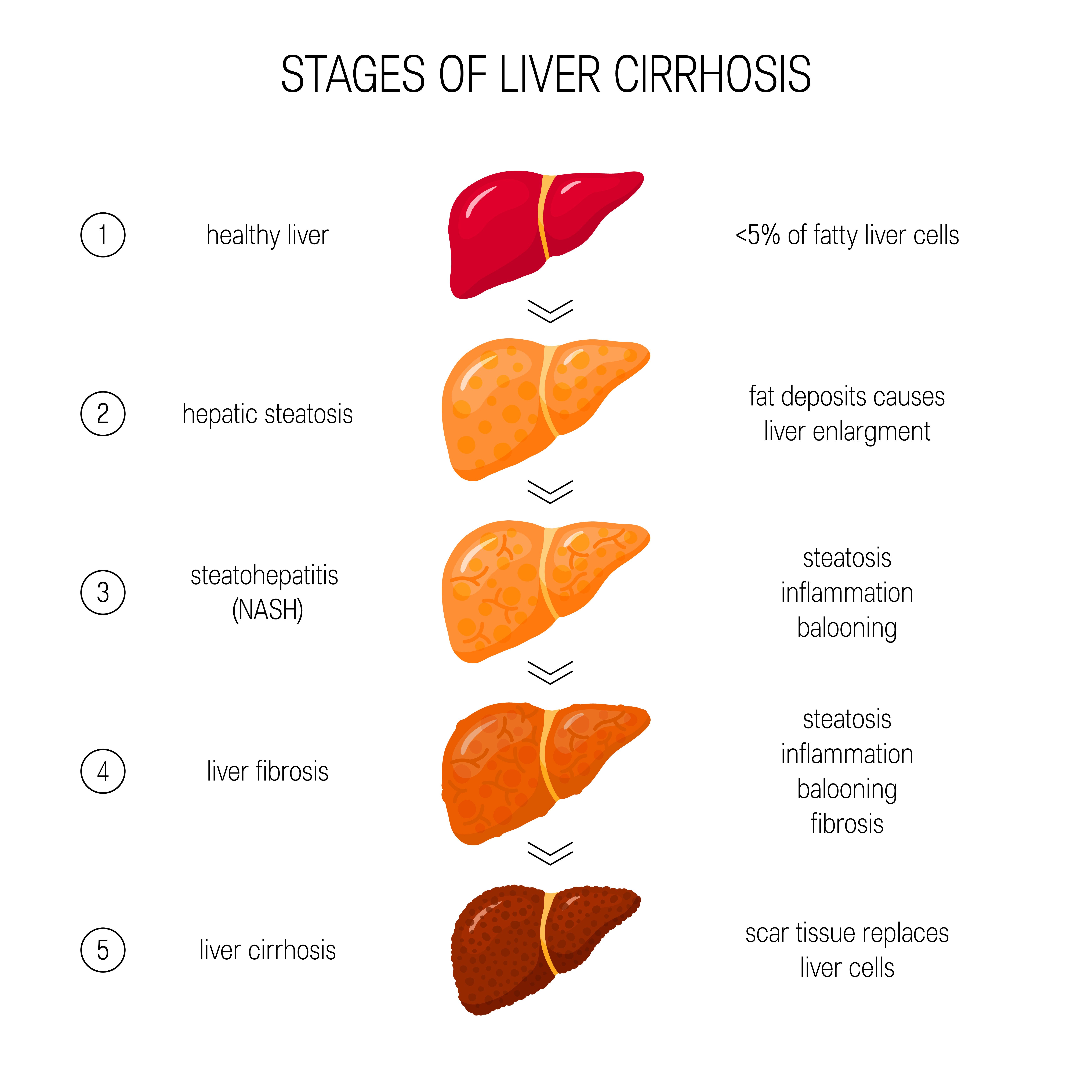
NASH is a progressive form of nonalcoholic fatty liver disease (NAFLD) characterized by inflammation, hepatocellular damage, and underlying fibrosis. It is often associated with obesity, diabetes, and metabolic syndrome, thereby increasing the risk of cardiovascular disease. The rising incidence of NASH has become a major public health issue, and experts predict it will become the leading cause of liver transplantation.
People who are overweight, have type 2 diabetes, or have a family member with NAFLD are at higher risk of developing the disease, also known as silent disease with few symptoms. NAFLD is a broad term that refers to a range of liver diseases that affect people who do not drink heavy alcohol and can lead to cirrhosis, liver cancer, and liver failure.
Although lifestyle changes (such as weight loss and exercise) are recommended as first-line treatments for NASH, they may not be sufficient to meet the needs of all patients. The FDA has not yet approved a drug to treat NASH, highlighting the urgent need for effective therapies to combat this debilitating disease.
Rohit Loomba, MD, PhD, of the University of California, San Diego School of Medicine, and colleagues conducted a study to identify a potential new treatment for patients with NASH and its associated fibrosis.Results published in June 2023 New England Journal of Medicineshowed that a drug called pegozafermin, which mimics the naturally occurring hormone fibroblast growth factor 21 (FGF21), could improve liver fibrosis and inflammation in patients with NASH.
Based on the study results, the FDA granted breakthrough status to pegozafermin in late September. The drug’s manufacturer, San Francisco-based biotech company 89Bio, said in a press release that the breakthrough will facilitate planning for Phase 3 trials.
The company-sponsored randomized controlled trial studied the efficacy and safety of the FGF21 analog pegozafermin in patients with NASH. This study aimed to evaluate the effect of pegzafenamine on fibrosis improvement and NASH regression in patients with biopsy-proven non-cirrhotic NASH without worsening fibrosis.
The study involved 222 participants with biopsy-proven NASH and stage F2 or F3 fibrosis. The primary endpoints were improvement in fibrosis and resolution of NASH, with no worsening of fibrosis at 24 weeks. Safety was also assessed. Further studies are planned to assess the drug’s safety and efficacy in a prospective Phase 3 trial.
Results showed that pegozafermin treatment significantly improved fibrosis. A higher percentage of patients met criteria for improvement in fibrosis in the pegozafermin group compared with the placebo group. Specifically, compared with placebo, there was a 14-point difference in the 15-mg pegozafermin group, a 19-point difference in the 30-mg group, and a 20-point difference in the 44-mg group.
Likewise, a significantly higher percentage of patients in the pegozafermin group met criteria for NASH resolution without worsening of fibrosis in the pegozafermin group compared with the placebo group. Compared with placebo, there was a 35 percentage point difference in the 15 mg group, a 21 percentage point difference in the 30 mg group, and a 24 percentage point difference in the 44 mg group.
The most common adverse events associated with treatment with pegolzatemine are nausea and diarrhea.
These positive findings will help advance pegozafermin into Phase 3 development.
Mary E. Rinella, MD, of the University of Chicago Pritzker School of Medicine, warned in an accompanying editorial that durability of treatment has been an issue with other FGF21 analogs and that anti-drug antibodies may be an issue with Pegozafermin. She noted that NASH is a chronic disease, so treatment may need to continue for years, similar to the treatment of type 2 diabetes.
“While the planned 24-week open-label extension of this trial will provide insight into the durability of responses to pegolzafermine, even 48 weeks may be too short to assess the durability of responses for this or any other FGF21-based therapy. Sex” Rinella wrote.
