The Ministry of Health will invest €1.9 million during the 2023-2024 season to immunize all susceptible children with nirsevimab against respiratory syncytial virus (RSV). Nirsevimab protects babies from RSV infection during their cycle season.
RSV causes a substantial annual burden of disease, including infections, hospital admissions, and ICU admissions, in the youngest children, especially premature infants, infants with underlying medical conditions, and infants less than six months healthy.
RSV is the leading cause of bronchiolitis, a disease consisting of inflammation of the bronchi and bronchioles, which accounts for 80% of respiratory illnesses and hospitalizations in children under 1 year of age. It is estimated that 10% of children develop the disease, of which 1-2% require hospitalization, and 10% of these children require admission to the ICU.
The disease is more common and more significant in premature or severely ill children, but because it is a very common disease, most people who become infected are healthy children. The most common complication of acute infection is respiratory distress, often requiring oxygen or other respiratory support measures.
Also, small children may have difficulty eating. Other microbial infections may also be seen, mainly otitis media or pneumonia. In addition to short-term complications, RSV infection in the first few months of life has been associated with asthma and wheezing episodes (“wheezing” difficulty breathing).
The Ministry of Health will immunize all children in the following groups with nirsevimab during the 2023-2024 season. for children with medical conditions o Conditions considered at risk due to severe RSV infection: premature infants less than 35 weeks and up to 12 months; congenital heart disease with severe hemodynamic impact up to 24 months.
Also, in cases of bronchopulmonary dysplasia, up to 24 months. Other complications, such as severe immunosuppression, inborn errors of metabolism, severe neuromuscular or pulmonary disease, genetic syndromes with associated respiratory problems, Down syndrome, cystic fibrosis, and patients on palliative care for the longest duration 24 months long.
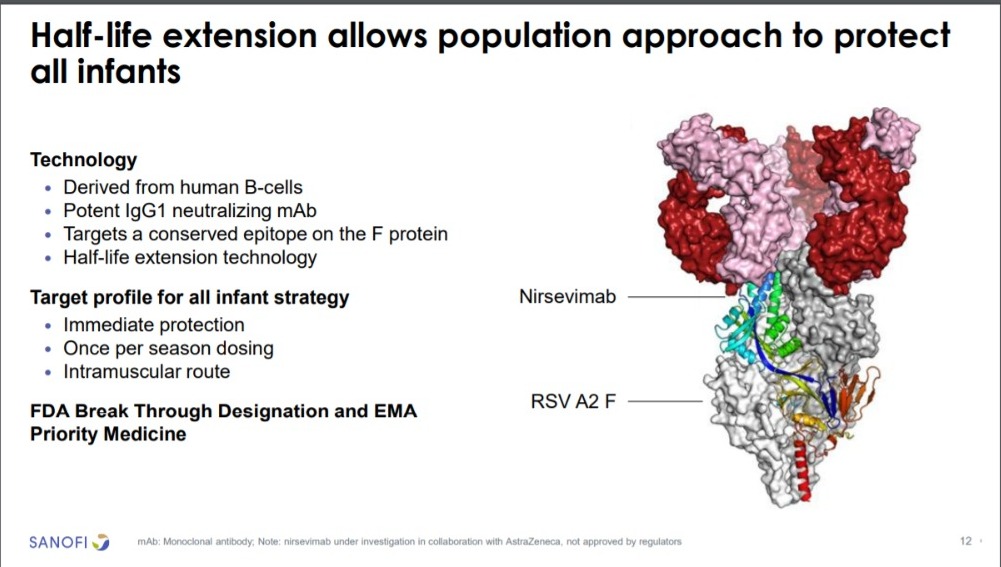
in healthy children: All persons 6 months of age or older or born during a VRS season. They are all children born between April 1, 2023 and March 31, 2024. In October 2022, the monoclonal antibody nirsevimab was authorized in the European Union and has been proven to be safe and effective for disease prevention.
This prophylaxis is especially effective for the most severe forms and the duration of protection covers almost the entire season of the RSV cycle, ie it can be used to prevent disease in all young children. The drug is administered as a single intramuscular injection and can be safely and effectively administered from birth.
For all these reasons, public health professionals and the Pediatric and Vaccine Society recommend that all baby use.
The Ministry is purchasing the drug (for which a total of 1.9 million euros has been allocated) and organizing events, and will inform professionals and the public where it will start, tour and administer. Protecting children from this disease benefits them, their families, and society as a whole.
Readings: 100