patient characteristics
The enrollment period is from July 9, 2019 to June 26, 2020, and the deadline for analyzing data is August 8, 2022. A total of 23 patients with advanced HCC were screened, of whom 3 were ineligible (Child-Pugh score > 6) (n= 1); non-measurable lesions (n= 1); ≥ 50% liver occupied (n= 1)). Finally, we enrolled a total of 20 eligible patients in the Phase 1 dose-reduction and Phase 2 dose-expansion cohorts (Figure 1). Patient characteristics are shown in Table 1. The median age of enrolled patients was 60.5 years; of them, 75% were male. Sixty percent of patients had a baseline ECOG performance status of 1. This study included 18 (90%) hepatitis B virus (HBV)-infected patients and 1 (5%) hepatitis C virus (HCV)-infected patient. Extrahepatic metastases were present in 19 (95%) patients and macrovascular invasion was present in 2 (10%) patients. Patients were heavily pretreated, and all had received prior systemic therapy, including sorafenib (19 (95%)) and lenvatinib (1 (5%)). During the study period, 17 of 20 patients (85%) discontinued treatment; of these, 16 (80%) discontinued due to disease progression or symptom worsening, and 1 (5%) discontinued due to withdrawal of consent. As of the data cutoff, 3 patients have completed 52 cycles of treatment with GT90001 combined with nivolumab and safety follow-up. The last patient’s last visit was April 28, 2022.
DLT
During the dose-tapering phase, only 1 of the first 6 patients required dose interruption between Cycles 1 and 2 due to sepsis (considered unlikely to be related to the study drug) and renal impairment (considered likely to be related to the study drug). Research drug related). One patient reported grade 3 platelet count reduction without active bleeding. The other 4 patients experienced grade 2 or less TEAEs within the first 28 days of treatment. Generally, GT90001 7.0 mg/kg was well tolerated, and no DLTs were observed in the 7.0 mg/kg cohort. Therefore, no dose reduction is required and GT90001 7.0 mg/kg is confirmed as RP2D when used in combination with nivolumab.
Safety and tolerability
All 20 patients treated with GT90001 plus nivolumab were evaluated for safety. Table 2 shows a summary of all grade and grade 3 or 4 TEAEs in the safety population. All patients (100%) experienced TEAEs (all causality); of these, 15 (75%) patients experienced a protocol-defined AESI. The most common TEAEs (all causality) in more than 20% of patients were decreased platelet count (11 of 20 patients (55%), including grade ≥ 3 in 3 (15%)), pruritus (9 (55%)) 45%) of 20 patients, 1 case ≥ grade 3), rash (8 cases (40%), 2 cases ≥ grade 3), peripheral edema (7 cases (35%); 1 case ≥ grade 3), Abdominal distension (5 cases (25%))), elevated ALT/AST (5 cases (25%); 1 case ≥ grade 3), fatigue (5 cases (25%)), epistaxis (5 cases (25%) ), cough (4 cases (20%)), constipation (4 (20%)) and dizziness (4 (20%)). A total of 11 patients (55%) experienced grade 3 or 4 AEs, the most common of which was decreased platelet count (15%). Grade 3 or 4 AEs were considered treatment related in 6 (30%) patients and included rash (n= 2), decreased platelet count (n= 3), AST increases (n= 1). All of these treatment-related grade 3 or 4 events resolved with supportive care.
A total of 7 SAEs were reported in 5 (25%) patients, and these events included hepatitis (n= 1), jaundice cholestasis (n= 1), hyperamylaseemia (n= 1), hyponatremia (n= 1), sepsis (n= 1), renal impairment (n= 1), and central nervous system metastases (n= 1). Of these, hepatitis, hyperamylaseemia, and renal impairment were judged to be treatment related (15%). Throughout the study, only 2 (10%) patients required dose reduction due to grade 3 platelet count decline, which was considered potentially related to GT90001 (Appendix file 1: Table S2). Therefore, the dose of GT90001 was reduced from 7.0 mg/kg to 4.5 mg/kg and the dose of nivolumab was suspended. In addition, nivolumab was discontinued in 1 patient because of rash and in another patient because of elevated ALT/AST and nephropathy. One patient discontinued GT90001 due to a decrease in platelet count. Nine (60%) patients discontinued treatment with GT90001 and nivolumab due to TEAEs (Appendix 1: Table S2). The most common cause was decreased platelet count (2 patients). In all other cases, supportive care is sufficient to reduce severity to grade 1 or less. There were no TEAE-related treatment discontinuations or deaths.
Although most patients had elevated systolic and diastolic blood pressure (Appendix file 1: Figure S2), only 3 patients were recorded as hypertensive adverse events according to NCI-CTCAE 5.0 (Table 2). No significant association was found between changes in blood pressure and response to study drug treatment (data not shown).
A total of 6 patients developed irAEs requiring steroid treatment. Among them, patient 1 (Pt1) developed a rash, which recovered after topical deoxymethasone ointment (twice daily for 4 weeks) and oral prednisolone (15-30 mg/day for 2 weeks). However, Pt2’s rash persisted even after 7 weeks of topical deoxymethasone and 1 year of oral prednisolone (5-30 mg/day). Additionally, one patient reported two episodes of suspected pneumonia and recovered after oral dexamethasone (4 mg, 7 days) and prednisolone (2-30 mg/day, 1 month). Three additional patients received dexamethasone for diabetes insipidus (subcutaneous, 4 mg), post-treatment sequelae of brain metastases (intravenous, 5 mg), and oral ulcers (topical, as needed for 2 weeks), all of which resolved.
effectiveness
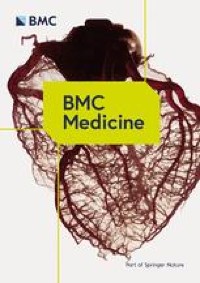
In Phase 1b and Phase 2, a total of 20 patients were enrolled to receive GT90001 at the 7.0 mg/kg dose level and were evaluable for efficacy. According to investigator assessment, target lesion size decreased in 9 (45%) patients (Fig. 2A). By investigator assessment, objective response was observed in 8 of 20 patients (40%; 95% CI, 19.1% to 63.9%); of these eight patients, all achieved a PR. Reassessment was performed on patients at week 4 when PR or CR was first reported as part of the workup for response confirmation. Six (30%) patients achieved confirmed PR (Table 3), with a confirmed ORR of 30% (95% CI, 14.6% to 51.9%). Among the 6 respondents, 5 (83.3%) patients had a DOR of more than 6 months, and 4 (66.7%) patients had a DOR of more than 12 months. The median DOR (NR; 95% CI, 7.39 months to NR) was not reached, and the median TTR was 1.91 months (95% CI, 1.17 to NR). Treatment times for all patients are shown in Figure 2B. Optimal response time to SD (≥ 16 weeks) was reported in 2 (10%) patients. Overall, confirmed DCR for SD ≥16 weeks was 40% (95% CI, 21.9% to 61.3%). The number of PFS events at data cutoff was 17, and the median PFS was 2.81 months (95% CI, 1.71 to 9.33 months). PFS at 6 months and 12 months were 35% (95% CI, 15.7% to 55.2%) and 25% (95% CI, 9.1% to 44.9%), respectively (Figure 3).
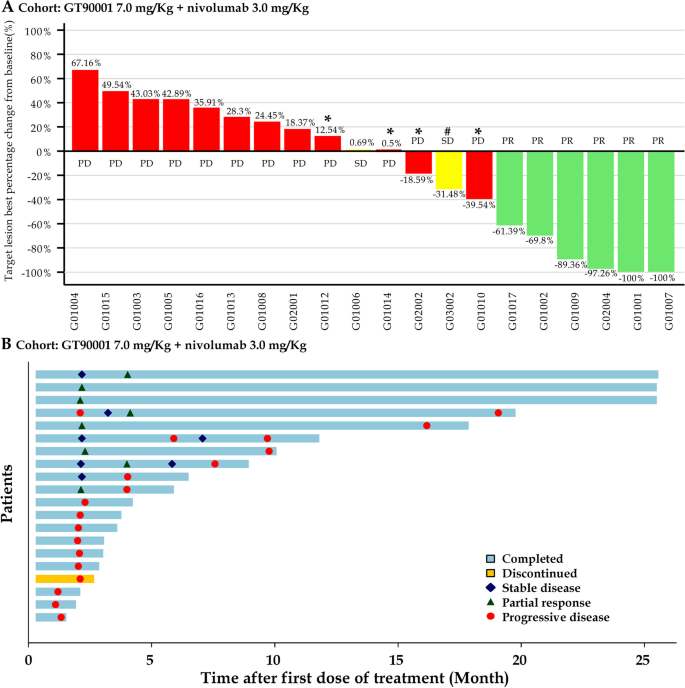
tumor response. arrive Waterfall plot of maximum percent change from baseline in tumor size for each patient, as measured by Response Evaluation Criteria in Solid Tumors (version 1.1). *: Patients whose PD status was recorded due to the development of new lesions. #: Patients with SD status recorded due to unconfirmed PR. Second Response time and response duration.Each bar represents a patient
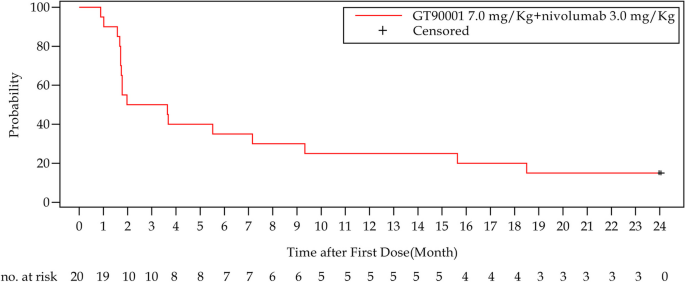
Kaplan-Meier curve of progression-free survival
One patient (Pt2, 54-year-old male) was diagnosed with pseudoprogression after GT90001 nivolumab exposure (Figure 4A). The patient suffered from multiple intrahepatic and extrahepatic metastases and started study treatment on August 5, 2019. After two cycles of treatment (September 2, 2019), the CT scan showed that the liver lesions had expanded. Laboratory tests confirmed increases in alpha-fetoprotein (AFP) and liver function indicators (AST and bilirubin) during pseudoprogression. As symptoms improved, the patient underwent postprogression therapy. Starting from the 10th week (October 14, 2019), the liver lesions showed necrosis, and the abdominal tumors continued to shrink, accompanied by a decrease in AFP and AST levels. At the one-year follow-up examination (July 2020), a CT scan showed high-density lesions, raising suspicion of tumor progression. In March 2021, the patient’s CT scan showed progression of the disease, showing expansion of liver lesions. Therefore, biopsies were collected for diagnostic purposes and demonstrated HCC recurrence. The patient underwent ablation therapy. Since then, the patient has shown no signs of progression and has been free of recurrence for more than 6 months. During treatment, I developed a rash after GT90001 nivolumab exposure. To control toxicity, he used topical dexamethasone ointment twice daily for 7 weeks, followed by 5-30 mg of prednisolone daily (February 4, 2020, to February 3, 2021), but the rash continued to fluctuate , until the end of follow-up. Above. Another patient (Pt6, 52-year-old male) with lung metastases started study treatment in December 2019 and achieved optimal response to SD. However, follow-up CT images showed that the lung lesions had gradually expanded since February 2020 (Figure 4B). Subsequently, the patient underwent surgical resection of lung metastases in November 2020 and had no recurrence for more than 6 months. Pt1 (60-year-old male) with lung metastasis has been studied for treatment since July 2019 and achieved the best response of PR. CT scan showed possible recurrence of HCC (lesion enlargement from 10 mm to 17 mm) (Figure 4C). Surgery confirmed the suspicion of HCC recurrence. In addition, the combination of GT90001 and nivolumab showed a significant long-tail effect, as 8 subjects were still alive as of the 19th.th April 2023. After the trial was completed, 6 of them did not receive further systemic anti-tumor treatment. Three patients did not receive any other anti-tumor treatment and are still in a progression-free state.
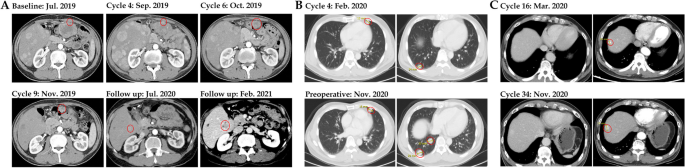
CT images of representative cases. arrive Patient 2 developed pseudoprogression after 4 cycles of GT90001 plus nivolumab and achieved partial response in the 6th cycle. Second Patient No. 6 with lung metastases was in stable condition during treatment, but at the same time, progressive expansion of lung lesions occurred in February 2020. After surgical removal of the lung lesions in November 2020, the patient had no recurrence for more than 6 months. C Patient 1 with lung metastases achieved PR on treatment but developed HCC recurrence