study options
After a literature search, we selected 16 randomized controlled trials involving 4278 adults with chronic hepatitis B treated with ETV, TDF, or TAF (Fig. 1 ). Data on renal function and skeletal changes were examined.
Learning features
One of the RCTs compared all three drugs, while the other fifteen trials compared only any two of the three drugs. Patients in all studies were from Asia, including China, South Korea and Japan. Therefore, the study results represent a limited racial group. However, they do provide clinical evidence of adverse effects of different drugs on renal function and bone. The basic characteristics of these studies are summarized in Table 1.
Network structure
In a network meta-analysis conducted, eGFR, creatinine, bone mineral density, and serum phosphorus concentration were examined in relation to renal function. In the network geometry shown in Figure 2, TAF, TDF, and ETV are represented as nodes, and the corresponding comparisons are represented as links between nodes. The size of the blue nodes is proportional to the number of patients using the drug, while the width of the black line is proportional to the number of studies comparing the two drugs. As shown in Figure 3, the most studies performed to date relate to eGFR. Studies comparing the effects of TDF and ETV on BMD were not included, but studies comparing other indicators and drugs were included.
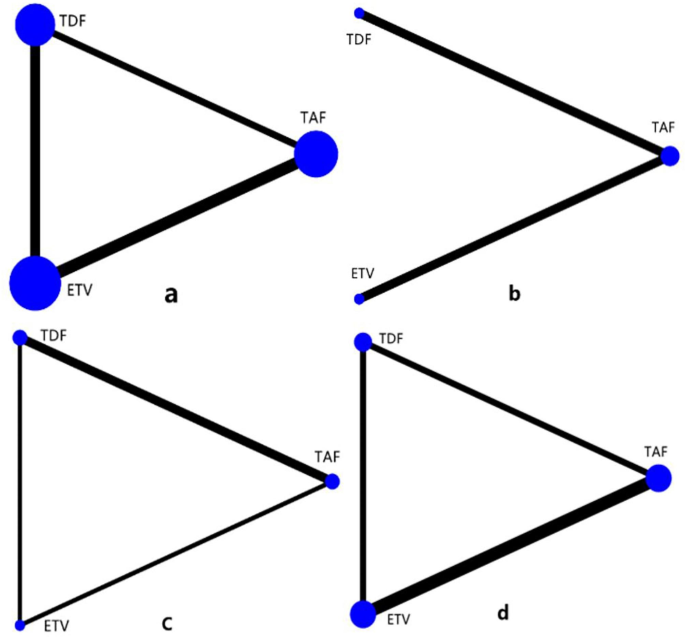
Geometry for network meta-analysis. The size of the blue nodes is proportional to the number of patients using the drug, while the width of the black line is proportional to the number of studies comparing the two drugs.Figure ad represents studies of eGFR, bone mineral density, creatinine and serum phosphorus respectively.
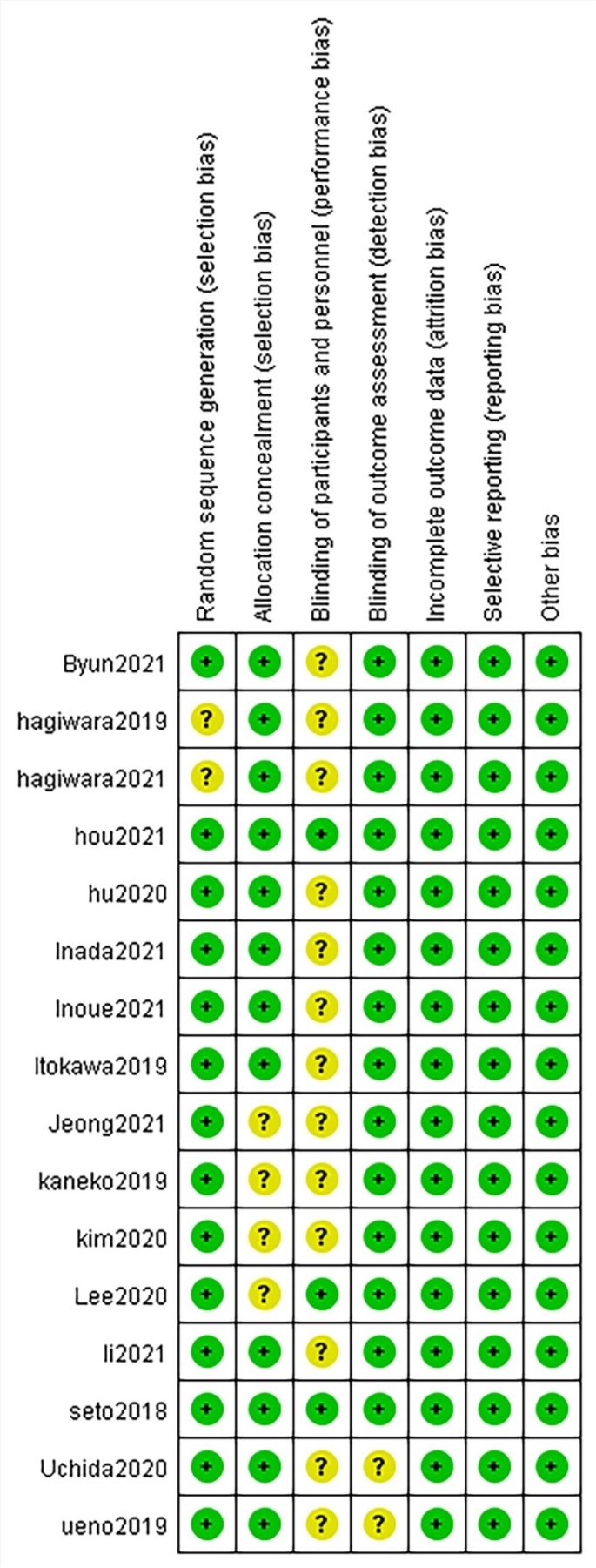
Bias risk summary.Green indicates low risk, yellow indicates unclear risk, and red indicates high risk
risk of bias
Two authors independently assessed all included studies using the Revman5.4 risk of bias tool (Fig. 3). The risk of bias was generally low and all studies were included in the systematic review.
Comprehensive results
renal events
The impact of ETV and TAF on the reduction of eGFR is less than that of TDF (SMD=-3.60; 95%CI: -1.94~-5.26 and SMD=-4.27; 95%CI: -2.62~-5.93, respectively). In contrast, the effects of ETV and TAF on eGFR reduction were not statistically significant (SMD=-0.67; 95%CI: -2.10~0.75) (Fig. 4a).
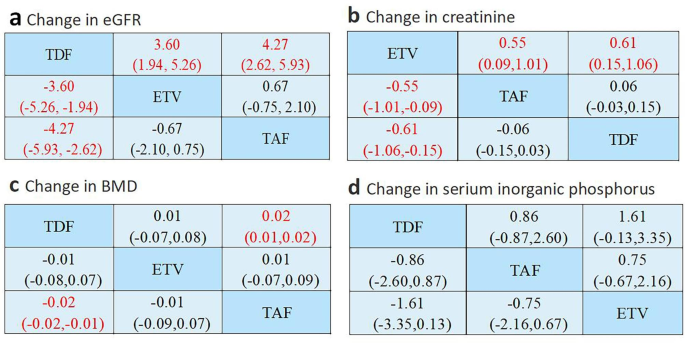
Network meta-analysis comparison of side effects. Data in the column-defined treatment were SMD (95% CI) compared to the row-defined treatment. Taking SMD=0 as the standard, a negative value indicates a strong downward effect or a weak upward effect on the index, and a positive value indicates a weak downward effect or a strong upward effect on the index. (a) Changes in eGFR, (b) changes in creatinine, (c) changes in bone mineral density, and (d) changes in serum phosphorus
Analysis of the creatinine data showed that there was a significant difference with TAF (SMD = -0.55; 95% CI: -0.09 to -1.01) and TDF (SMD = -0.61; 95% CI: -0.15 to -1.06). At the same time, there was no statistical significance when comparing the effects of TAF and TDF on the increase in creatinine (SMD=0.06; 95%CI: -0.03~0.15) (Figure 4b).
As shown in Figure 5 , TAF exhibited the lowest probability of eGFR reduction (SUCRA 8.8%), followed by ETV (SUCRA 41.2%). In contrast, TDF showed the highest probability of eGFR reduction (SUCRA 100.0%). Regarding creatinine, ETV was least likely to increase creatinine (SUCRA 0.6%), whereas TAF (SUCRA 54.7%) and TDF (SUCRA 94.7%) were more likely to increase creatinine, respectively. Taken together, these data suggest that TDF has a greater detrimental effect on renal tissue than TAF or ETV.
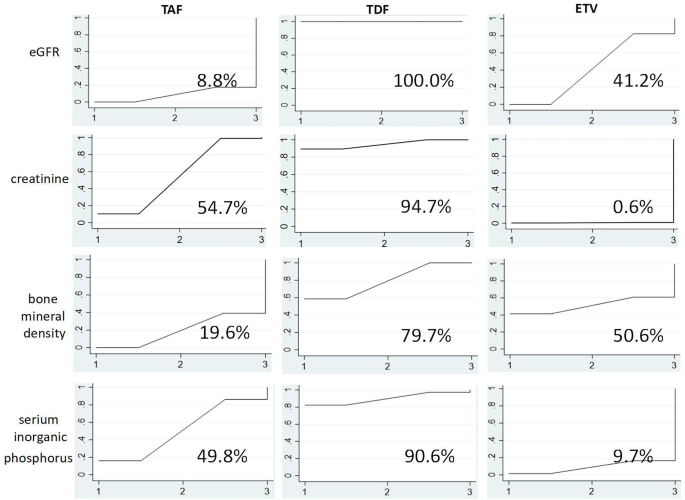
SUCRA Side Effect Chart. The figure shows the probability of the impact of the three drugs on eGFR, creatinine, bone density, and blood phosphorus before and after administration.According to the level of area under the curve (SUCRA), the larger the area, the greater the index change value.
Bone events
To examine the effects of TAF, TDF, and ETV on bone mineral density, differences in lumbar spine bone mineral density before and after treatment in 1,419 subjects were analyzed in four studies. Differences in serum phosphorus before and after treatment in 926 subjects from eight studies were also analyzed.
As shown in Figure 4c, TAF significantly reduced bone mineral density than TDF (SMD=-0.02; 95%CI: -0.01~-0.02). In contrast, there was no significant difference in BMD between ETV and TDF or between ETV and TAF (SMD = -0.01; 95%CI: -0.08 ~ 0.07 and SMD = 0.01; 95%CI: -0.07 ~ 0.09, respectively). Therefore, TAF has a smaller effect on bone density than TDF, and ETV is not comparable to TAF and TDF. At the same time, there was no statistical difference in the serum phosphorus levels of the three drugs (Fig. 4d).
As shown in Figure 5, TAF has the lowest possibility of reducing BMD (SUCRA 19.6%), followed by ETV (SUCRA 50.6%). In contrast, TDF had the highest probability of reducing BMD (SUCRA 79.7%). Regarding blood phosphorus, ETV has the lowest probability of reducing blood phosphorus (SUCRA 9.7%), followed by TAF (SUCRA 49.8%). TDF has the highest possibility of reducing serum phosphorus (SUCRA 90.6%). Based on these two sets of data, TDF has a greater adverse effect on bone tissue than TAF or ETV.
Deviation analysis
Use Stata 15 software to generate a funnel plot as shown in Figure 6. Each point in the triangle in the figure is roughly symmetrical to the central axis, indicating that publication bias is controllable.
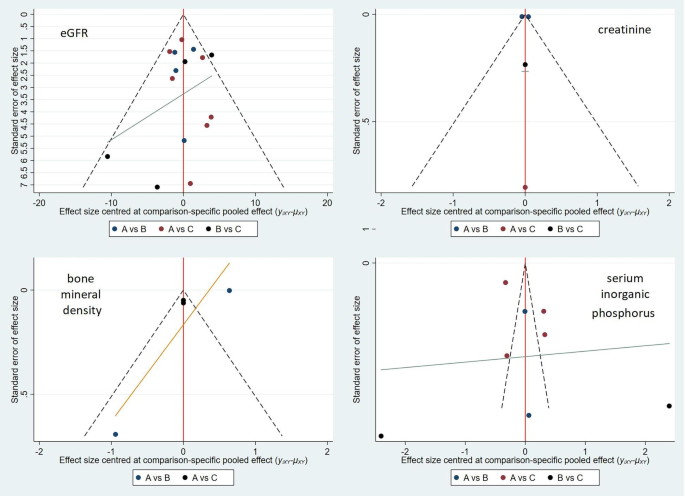
Comparison of each side effect Adjusted funnel plot to indicate bias
Subgroup analysis of medication time
Subgroup analysis was performed on the effects of TDF and ETV on eGFR and serum phosphorus according to administration time. Figure 7 shows renal function and bone tissue damage in patients treated with TDF and ETV for different durations.
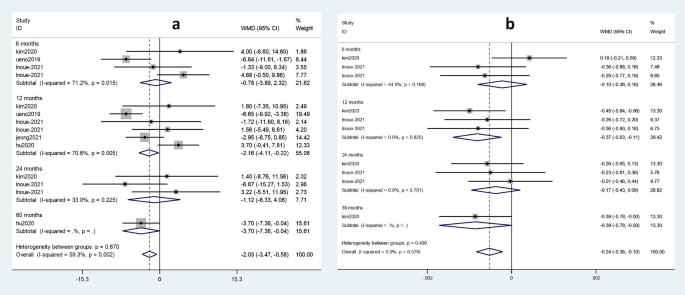
Forest plot of TDF and ETV subgroup analysis of eGFR and serum phosphorus according to treatment duration. arrive) Effect of TDF on eGFR compared with ETV. Second) Effect of TDF on serum phosphorus compared with ETV
The effect of the two drugs on eGFR was at 6 months (weighted mean difference (WMD) = -0.78; 95%CI: -3.89 ~ 2.32) or 24 months (WMD = -1.12; 95%CI: -6.33) No statistical significance. ~4.08) (Fig. 7a). However, comparison of drug efficacy after 12 months (WMD = -2.16; 95%CI: -4.11 ~ -0.22) and 60 months (WMD = -3.70; 95%CI: -7.36 ~ -0.04) showed that TDF showed a greater adverse effect on eGFR than ETV at 60 months.
As shown in Figure 7b, no statistical effect on serum phosphorus was observed after 6 months (WMD=-0.10; 95%CI: -0.30~0.16) or after 24 months (WMD=-0.17; 95) for both drugs. significant impact on. %CI:-0.43~0.09). In contrast, when the medication was taken for 12 months (WMD=-0.37; 95%CI: -0.63~-0.11) or 36 months (WMD=-0.39; 95%CI: -0.78~0.00), there was Statistically significant effects were observed on changes in serum phosphorus. Therefore, compared with ETV, TDF significantly reduced serum phosphorus levels at 36 months than at 12 months.

